
The heart procedure, performed last August, uses the Abbott TriClip™ transcatheter edge-to-edge repair (TEER) system. The AGH cardiac team is the first in the state of Pennsylvania to implant the system. (Photo courtesy of Abbott)
Cardiac Surgeons Successfully Implant First-of-its-Kind Device to Repair Leaky Heart Valve
Surgeons at Pittsburgh’s Allegheny General Hospital were the first in Pennsylvania to accomplish the procedure, using the newly approved device to repair a leaky tricuspid valve.
PITTSBURGH—Last August, a cardiac team at Allegheny General Hospital (AGH) reported that it successfully implanted a newly approved, first-of-its-kind device to repair a leaky tricuspid valve, which controls blood flow between heart chambers.
The heart procedure, which uses the Abbott TriClip™ transcatheter edge-to-edge repair (TEER) system, was led by David Lasorda, M.D., Rachel Hughes-Doichev, M.D., and Walter McGregor, M.D., of the Allegheny Health Network (AHN) Cardiovascular Institute.
Allegheny General Hospital is the academic flagship facility of Allegheny Health Network (https://www.ahn.org/), and its cardiac team is the first in the state of Pennsylvania to implant the TriClip system.
The tricuspid valve sits between the right ventricle and the right atrium and is made of three flaps of tissue. When the valve doesn’t close properly, blood flows backward in the heart. This condition is called tricuspid valve regurgitation, a malady that affects about 1.6 million Americans, including tens of thousands in Western Pennsylvania.
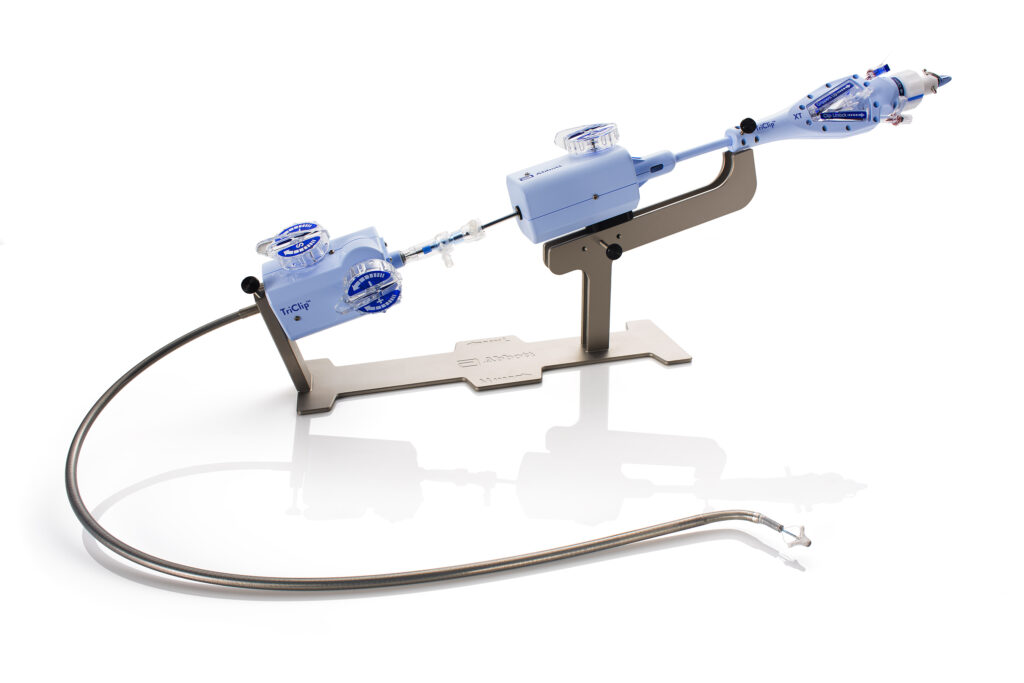
Delivered through a vein in the leg, TriClip’s TEER technology works by clipping together a portion of the leaflets—or flaps of tissue—to repair the tricuspid valve and help blood flow in the right direction without the need for open-heart surgery. (Photo courtesy of Abbott)
The TriClip system is intended to improve the function of the tricuspid valve.
“Previously, patients who suffered with tricuspid regurgitation did not have many surgical intervention options or medical therapies that would significantly relieve associated symptoms, which can be debilitating,” McGregor said in a release from Allegheny Health Network. “We’re extremely pleased to be the first medical center in the state to offer this new, minimally invasive treatment, which has proven to yield positive health outcomes and ultimately improve the quality of life for this patient population.”
When blood flows backward into the right atrium of the heart, blood volume and pressure on the right side of the heart increases, often leading to an enlarged heart. Other tricuspid regurgitation (TR) symptoms include shortness of breath, fatigue, abdominal swelling, enlarged liver, active pulsing in the neck veins, and swollen legs, ankles, and feet. If left untreated, TR may progress into conditions such as atrial fibrillation and heart failure, according to the American Heart Association.
“TriClip is an exciting advancement in cardiovascular therapies that provides a much-needed solution for patients diagnosed with such a complex heart condition,” said Stephen Bailey, M.D., cardiothoracic surgeon and chair of AHN Cardiovascular Institute, in the release.
The TriClip is delivered to the heart via a catheter inserted through the femoral vein in the leg. It works by clipping together a portion of the leaflets of the tricuspid valve. By clipping the leaflets together, the heart valve properly closes, reducing the backflow of blood. The TriClip also varies in size, enabling a precision fit regardless of the size of the patient’s heart valve.
On average, patients who undergo this procedure spend just one day in the hospital before returning home, according to AHN.
The TriClip TEER system received approval from the U.S. Food and Drug Administration in April 2024, following published research demonstrating its overall safety and efficacy.
Cardiothoracic surgeons and researchers from the Minneapolis Heart Institute led a clinical trial, TRILUMINATE Pivotal, in which Allegheny Health Network participated. Patients suffering from tricuspid regurgitation were enrolled in the trial across 65 sites throughout the U.S., Canada, and Europe.
The study enrolled more than 500 patients, with half of them in the control group (receiving medical therapy alone), and the other half in the group that received the TriClip. Results were published in the New England Journal of Medicine (DOI: 10.1056/NEJMoa2300525).
Results of the study are reported to have shown that the experimental group experienced significantly improved quality of life, and those improvements were sustained at both the 30-day and one-year marks. Patients in the TriClip group also saw more improvement in their tricuspid regurgitation, and the device itself was found to be a safe therapeutic intervention.

The TriClip varies in size, enabling a precision fit regardless of the size of the patient’s heart valve. (Photo courtesy of Abbott)
The average age of those enrolled in the TRILUMINATE Pivotal trial was 78. Over half of the patients enrolled were women, a cohort representative of the U.S. patient population diagnosed with tricuspid regurgitation. Many of these individuals, due to age and comorbidities, are not suitable candidates for more invasive open-heart procedures. That’s why the minimally invasive TriClip system is such an attractive option, explained McGregor.
Allegheny General Hospital is an award-winning, nationally-recognized center for the treatment of complex cardiovascular diseases, including the treatment of structural heart abnormalities and conditions.
In 2023, for the third consecutive year, the hospital was recognized as a Mitral Valve Repair Reference Center by the American Heart Association and Mitral Foundation. This distinction, awarded to just 21 programs nationwide, highlighted AGH’s excellence in treating degenerative mitral valve disease and made it the only Pennsylvania hospital with this prestigious recognition, according to Allegheny Health Network.
Orfit Industries, ZAP Surgical Collaborate to Advance Radiosurgery with Enhanced Patient Positioning
The partnership is reported to increase treatment precision by integrating Orfit’s HP PRO into the ZAP-X platform at 15 clinical sites worldwide.
WIJNEGEM, Belgium—Orfit Industries is collaborating with advanced radiosurgery technology company ZAP Surgical Systems, Inc., makers of the ZAP-X® Gyroscopic Radiosurgery® platform, in a partnership that is reported to be “demonstrating remarkable results in enhancing patient care and treatment precision.”
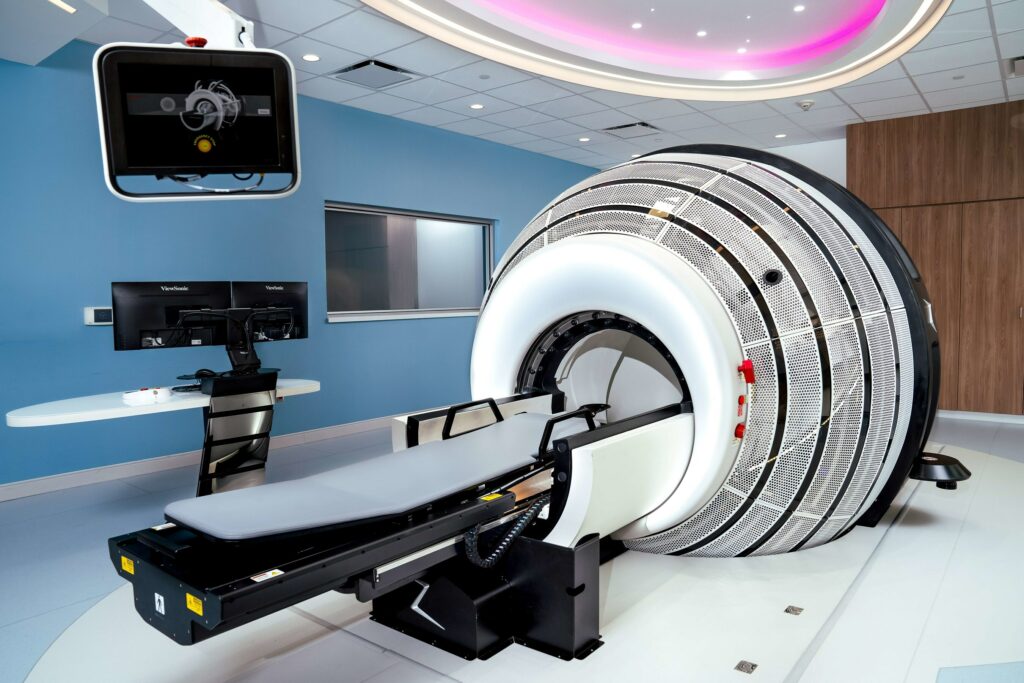
Orfit’s HP PRO is shown integrated into ZAP Surgical Systems’ ZAP-X® Gyroscopic Radiosurgery® platform. (Photo courtesy Orfit Industries/ZAP Surgical Systems/PRNewswire-PRWeb)
ZAP Surgical’s innovative ZAP-X, equipped with Orfit’s state-of-the-art HP PRO patient positioning and immobilization system, has been adopted by leading clinical centers worldwide, according to a release from Orfit Industries. This integration is said to ensure superior patient comfort and accuracy during treatments, with the goal to enable clinicians to deliver exceptional outcomes with confidence.
“The number of installations is rapidly growing worldwide, reflecting the increasing demand for this innovative solution,” the release stated.
After extensive validation and clinical use, the combined solution is reported to have proven its value in real-world settings. The success of the collaboration, according to Orfit, reflects the shared commitment of Orfit and ZAP Surgical to improving patient outcomes through cutting-edge innovation.
“Our collaboration with ZAP Surgical highlights Orfit’s dedication to driving innovation in patient positioning,” said Orfit Industries Head of Strategic Alliances Emilie Cuypers, in a statement. “With proven results at 15 active sites, and more installations on the horizon, we’re proud to play a role in advancing the future of radiosurgery.”
New Carotid Stenting System Receives FDA Approval for Carotid Revascularization
Contego Medical’s system is reported to make carotid stent procedures safer by capturing emboli that are not captured by traditional embolic protection systems.
RALEIGH, N.C.—A new carotid stenting system developed by Contego Medical is reported to streamline carotid stent procedures by reducing the number of steps required, while also making them safer by capturing emboli that are not captured by traditional embolic protection systems.
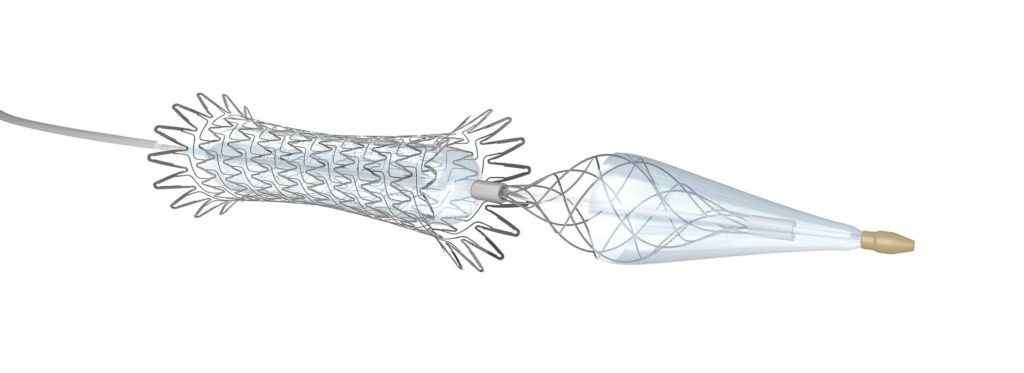
Contego Medical’s Neuroguard IEP® System is a unique 3-in-1 carotid stenting system that combines a high-performance stent, an integrated dilation balloon, and integrated filter. (Image courtesy Contego Medical)
Contego Medical, a developer of innovative medical devices for cardiovascular procedures, announced last fall that it received the U.S. Food and Drug Administration’s (FDA) premarket approval (PMA) of its Neuroguard IEP® System. Neurogard IEP is a unique 3-in-1 carotid stenting system that combines a high-performance stent, an integrated dilation balloon, and integrated filter.
Clinical studies of the Neuroguard IEP System, including the PERFORMANCE I Trial and PERFORMANCE II IDE Trial, are reported to have consistently recorded “unprecedented low event rates—zero major strokes, zero neurologic deaths, and zero stent thrombosis at 30 days and 1 year,” according to a release from Contego Medical.
“FDA approval confirms the results of the clinical studies,” said William Gray, M.D., system chief of the Division of Cardiovascular Diseases at Main Line Health, and co-national principal investigator of the PERFORMANCE II trial, in a statement. “The innovative Neuroguard IEP system performs exceptionally well with the lowest one-year stroke rates ever shown for any type of carotid revascularization, thereby establishing a new standard of care for meaningfully reducing the risk of procedural and long-term stroke among patients with carotid artery disease.”
The Neuroguard IEP System uses Contego Medical’s clinically-proven Integrated Embolic Protection™ (IEP) technology, featuring a 40-micron filter integrated on a 6 French delivery catheter. The size of the integrated filter can be adjusted by the operator to custom fit each patient’s unique anatomy. In addition, the pores on the filter are three to four times smaller than those on traditional filters, improving protection against stroke and cognitive impairment, the company said in the release.
The newest generation closed-cell stent is described as “highly conformable” and shows remarkable short and long-term durability. No thromboses, no clinically driven target lesion revascularization, and very low restenosis rates were reported at one year, according to the company.
“This FDA approval is a huge step forward for Contego and for patient care,” said Contego Medical CEO and Founder Ravish Sachar, M.D., in a statement. “The Neuroguard IEP System transforms how we approach patients with carotid artery disease by addressing the specific threats of microembolization while simultaneously reducing procedural steps, ensuring patients receive the highest level of protection throughout the procedure.
“The ongoing PERFORMANCE III trial is evaluating the Neuroguard IEP system with a next-generation direct transcarotid access and protection system, TCAR-IEP, to demonstrate advanced stroke protection regardless of vascular approach,” he added.
In 2023, the Centers for Medicare & Medicaid Services (CMS) issued a national coverage decision significantly expanding coverage for percutaneous transluminal angioplasty (PTA) of the carotid artery, concurrent with stenting performed with FDA-approved carotid stents and embolic protection devices.
“This decision and FDA approval of the Neuroguard IEP System positions Contego for ongoing growth,” the company stated in the release.
First Patients Treated with Neuroguard IEP System
In December, Contego Medical reported the successful completion of the first commercial cases with the newly FDA-approved Neuroguard IEP® System. John Gaughen, Jr., M.D., an interventional neuroradiologist at Centra Medical Group in Lynchburg, Virginia, and Srinivas Attanti, M.D., an interventional cardiologist at the University of Florida Health Leesburg Hospital in Leesburg, Florida, treated the first patients, according to a release from Contego.
“The Neuroguard System is very easy to use and streamlines the steps for treating our patients with carotid artery disease,” Gaughen said in a statement. “I believe this innovation will reduce the risk of stroke during and after carotid artery stenting procedures.”
New Wearable Device Monitors Joint Pain
Arthroba enables people—and their doctors—to follow their joint health in real time.
By Tess Malone
ATLANTA, Ga.—Samer Mabrouk started playing squash as an undergraduate at Georgia Tech. Ankle injuries were to be expected, and resting for a few days was all he needed to get back on the court. Now a research engineer in the School of Electrical and Computer Engineering at Georgia Tech, Mabrouk hasn’t put his racket down, but he gets injured more often—and rest isn’t enough anymore.

Omer Inan holds an early prototype of Arthroba. (Photo: Christopher McKenney)
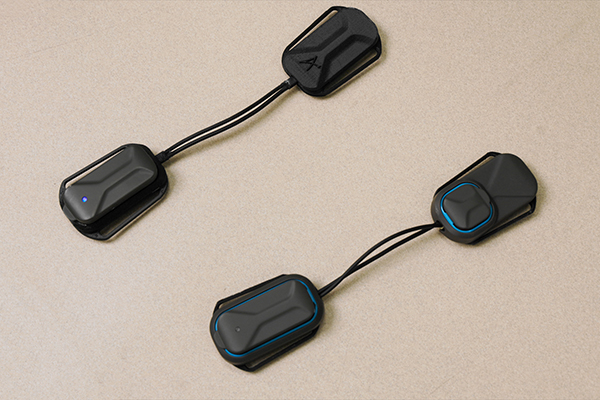
Arthroba is a lightweight, battery-powered device that can be easily worn around the knee. (Photo: Christopher McKenney)
Injuries like Mabrouk’s are common for active Americans. About 25,000 people sprain their ankles daily, and 25 percent of Americans regularly experience knee pain. But physical therapy is expensive and time-consuming, and online options for rehab aren’t precise enough to be effective.
Mabrouk and Omer Inan, Regents’ Entrepreneur and the Linda J. and Mark C. Smith Chair in the School of Electrical and Computer Engineering, have been working on a wearable, battery-powered device that monitors joint health and gives personalized strengthening exercises. Inan and Mabrouk’s new company, Arthroba, created a device of the same name that uses electrical sensors to track swelling and tissue damage in the knee, ankle, and other critical joints.
A healthy joint has a delicate balance of fluid between bone, tendons, ligaments, and cartilage—too little fluid can lead to arthritis and stiffness, while too much indicates swelling. When the fluid balance is off, people often experience pain and mobility problems. Arthroba uses bioimpedance analysis, or electrical sensing of the tissue, to determine if there is swelling or damage around a joint and if there is enough soft tissue to support the joint.
“Impedance is how much something impedes—or slows down—electrical current through that material,” Inan noted. “Bioimpedance looks at how much the electrical current is impeded as it flows through the body.”
When the composition of joint tissue changes because of swelling or injury, it can block that current. Arthroba uses four dry electrodes placed around the knee to quantify accumulated fluid and how it moves around the joint, allowing for more targeted physical therapy. The device can also evaluate and detect swelling and muscle tears, and it contains an inertial measurement unit that tracks the acceleration, rotation, and velocity of the joint. Together, they can unlock new insights into joint health and optimize treatment protocols.
Users can run the device during rehab exercises to avoid overuse. With its objective sensing, Arthroba can adjust therapy protocols in a personalized manner normally only achievable through frequent in-person assessments from highly trained therapists.
“Arthroba is comparable to body mass composition scales, but we measure a confined piece of your tissue,” Mabrouk said. “The changes we can see through the app correlate to many symptoms physiotherapists measure to recommend exercises.”
This data gets sent via Bluetooth to a mobile app that uses artificial intelligence to give exercise recommendations. Medical professionals can also use the data to design recovery processes.
Inan and Mabrouk have been working on this technology for years through grants from the Defense Advanced Research Projects Agency (DARPA) and the National Institutes of Health (NIH). Although Arthroba is designed for knee monitoring, the researchers have also worked with Georgia Tech baseball players on elbow-joint injuries, as well as patients with juvenile arthritis. They envision the technology being used not only for injury but also for preventive care.
“People want to keep playing tennis or pickleball without a lot of knee pain, injury, or risk,” Inan said. “Arthroba could be key to that.”
Tess Malone is a writer for Georgia Institute of Technology.
This article originally appeared in Georgia Tech Research News.
Source: https://research.gatech.edu/feature/arthroba
Film-Cast PTFE Catheter Liners Are Engineered for Flexibility, Superior Strength
To manufacture the liners, Zeus uses a proprietary film-cast process that is reported to help reduce surface imperfections and pinholes, a common issue with existing film-cast liners.
ORANGEBURG, S.C.—When Zeus, a global polymer extrusion and catheter manufacturer, recently introduced the latest addition to its StreamLiner series of ultra-thin-walled catheter liners, the company called them “the most flexible PTFE catheter liners ever produced by Zeus.”
The company’s StreamLiner™ NG catheter liners are reported to be ideal for traversing the most complex vasculatures, such as those found below the knee and above the neck, to deliver critical therapies.
Zeus uses a proprietary film-cast process to produce its new StreamLiner™ NG catheter liners with “significantly improved consistency when compared to existing film-cast liners, helping to reduce imperfections and pinholes commonly seen in other offerings,” the company said in a release. This consistency, combined with the exceptional sizing and tolerances of existing StreamLiner offerings, is said to result in an ultra-thin liner with remarkable flexibility, mechanical performance, and reliability.
“Given the flexibility film-cast liners provide, engineers prefer them for certain applications but face performance challenges due to surface imperfections and pinholes,” said Zeus Chief Technology Officer Suresh Sainath, in a statement. “Our new film-cast liner is the solution. By reducing surface imperfections and pinholes, StreamLiner NG can navigate the most tortuous paths and deliver superior strength and performance, significantly reducing these challenges.”
“For years, the Zeus StreamLiner series has set the standard for ultra-thin-walled, high-performance PTFE catheter liners,” said Zeus Chief Technology Officer Suresh Sainath, in a company release. “Now, we’re adding to this incredible product portfolio by introducing our first film-cast offering to the PTFE StreamLiner family, one that will raise the bar for what engineers can expect in performance from film-cast liners.”
Orthopedic Implant Manufacturer Receives U.S. FDA 510(K) Clearance of Medial Congruent Insert for Knee System
Maxx Orthopedics’ new tibial insert is “designed to give patients knee movement, in cruciate-retaining (CR) procedures, that mimics the natural knee,” the company’s CEO said.
NORRISTOWN, Pa.—A new medial-congruent insert for Maxx Orthopedics’ Freedom® Total Knee System is “designed to enhance total knee arthroplasty performance by incorporating features that better replicate natural knee function and motion,” according to a release from Maxx Orthopedics.
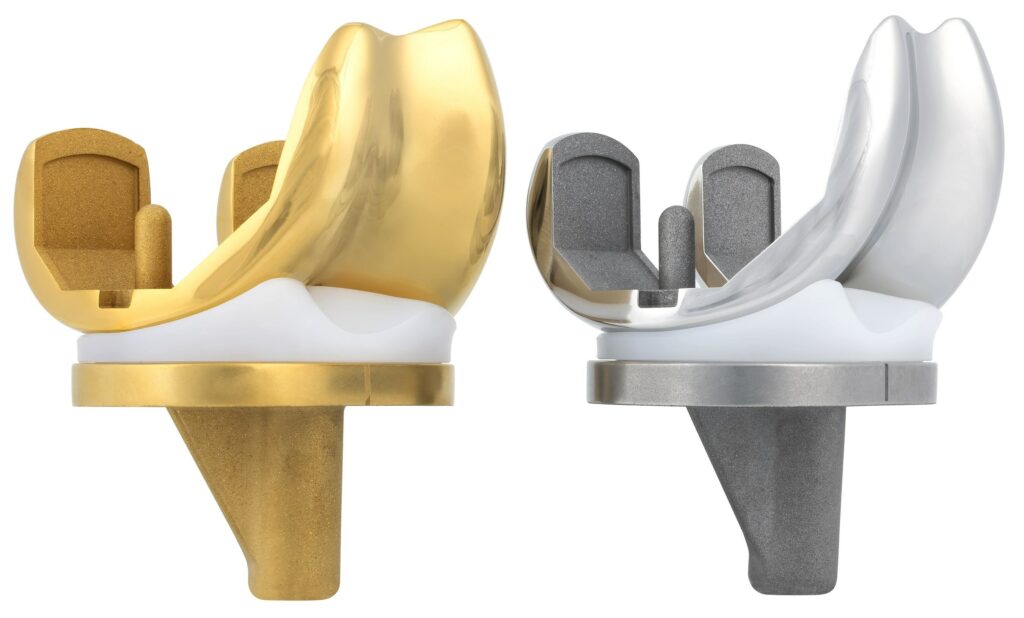
The Freedom Medial Congruent insert (Image courtesy Maxx Orthopedics/ PRNewswire-PRWeb)
Natural knee mechanics, with improved anterior sliding, lateral rollback, and external rotation, are among the features incorporated in the insert. So is medial stability, which ensures that the medial compartment remains congruent throughout the full range of motion. Another feature is “stability in extension, with a medial anterior lip to reduce anterior subluxation and enhance stability when the leg is in full extension,” the company said in the release.
Maxx Orthopedics, a global manufacturer of innovative orthopedic implants, recently received U.S. FDA clearance of its new Medial Congruent (MC) insert for the Freedom Total Knee System. The Freedom Medial Congruent, scheduled to be available for clinical use in early 2025, is said to represent “another pivotal step forward in delivering knee replacement technologies designed to improve patient outcomes and satisfaction.”
“The Freedom Medial Congruent insert reflects Maxx Orthopedics’ commitment to continuous innovation,” said Ashesh Shah, chief executive officer of Maxx Orthopedics, Inc., in a statement. ”The Freedom Medial Congruent insert expands the Freedom Knee portfolio by providing a tibial insert designed to give patients knee movement, in cruciate-retaining (CR) procedures, that mimics the natural knee.”
According to Maxx Orthopedics, the Freedom Total Knee System is widely recognized for its versatility and efficiency, offering a range of solutions for diverse patient needs.
“The addition of the Medial Congruent poly insert builds on this legacy, delivering a refined solution that balances knee function and patient mobility,” the release stated.
In addition to manufacturing the Freedom Knee System, Maxx Orthopedics also produces the Libertas® Total Hip System and Quick Recovery Solutions (QRS®).
“We develop and market innovative orthopedic medical devices with a focus on providing state-of-the-art implants and related solutions designed that best restore patient mobility, while accommodating lifestyle, anatomical, and economic needs,” the company stated in the release.
